ELISA
For Immunodiagnosis and Immunomonitoring
of Tropical Diseases
B.
C. Harinath & M. V. R. Reddy
Enzyme Linked Immuno Sorbant Assay
(ELISA) is a very sensitive and safe technique used in the detection of antigens
and antibodies was introduced by Engvall and Perlmann (1). The sensitivity of
this technique is comparable with that of Radio immunoassay with an added
advantage of safer use of non radioisotopic reagents and longer shelf life of
the same. It also eliminates the requirement of sophisticated isotope counters.
ELISA can be done in smaller laboratories and is adaptable to field conditions
as well.
The application of ELISA has been well reviewed (2,3). ELISA plays an
important role in the laboratory and in the field. The various components
involved in ELISA are a solid phase to which specific antigen/antibody is
coated, an antigen or antibody enzyme conjugate as probe as the case may be and
enzyme substrate. Solid supports used are polystyrene or PVC microtitre plates,
tubes or beads. Nitro cellulose paper or Cellulose Acetate Membrane are also
used as solid phases. Enzymes used for conjugation to anti immunoglobulins
include peroxidase, b-galactosidase, alkaline phosphatase, penicillinase, urease,
glucose oxidase. No enzyme fulfills all the criteria for an ideal label in EIA.
The influence of the solid phase on the enzyme should be minimal. Conjugation to
anti-immunoglobulin should be easy and conjugates should be active and stable.
These are the reasons for frequent selection of peroxidase in commercial
diagnostics. In our laboratory we have been successful in using penicillinase as
marker in EIA for tropical diseases. Penicillinase has also been successfully
used as an enzyme marker in hormone assays (4,5). Penicillinase (b-lactamase,
EC 3.5.2.6) has high turnover number of 1,60,000 and has been found to be
more sensitive than peroxidase, alkaline phosphatase or b- galactosidase (6).
The substrate (Penicillin V) used is not carcinogenic and other advantages of
using penicillinase are that the enzyme is quite stable and is not present in
biological fluids under normal conditions. Penicillinase activity can be
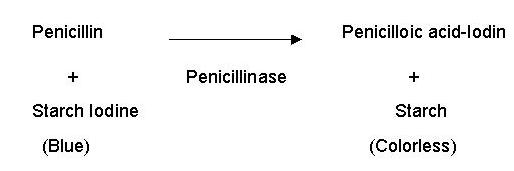
reliably estimated iodometrically. The assay involves decolourisation of blue
coloured starch-iodine-penicillin substrate. The result is assessed visually by
naked eye. The reaction may be stated as follows.

FILARIASIS
In our laboratory penicillinase ELISA system has been explored in
several ways such as Indirect, Direct, Sandwich, Competitive and Inhibition
ELISA for, detection of antibody, antigen and immune complexes in bancroftian
filariasis (9,10). Wuchereria bancrofti microfilariae excretory-secretory (Wb mf ES)
antigen was biosynthetically labeled using 14C-glucose
in the culture medium. The use of 14C-glucose
labeled Wb ES antigen in penicillinase- ELISA and Radio immunopolyethylene
glycol precipitation assay (RIPEGA) for detection of antibody showed that
penicillinase- ELISA was more sensitive than RIPEGA (11). Similarly inhibition
ELISA for detection of antigen was also compared with RIA using biosynthetically
labeled ES antigen and observed that ELISA was found to be requiring less antigen was simple
and inexpensive than RIA (12).
Indirect ELISA
Indirect
ELISA is useful for the detection of antibody using specific filarial antigen.
In this assay, the PVC plate was coated with antigen and the test sample
(serum/blood, hydrocele fluid, urine, etc.) was added to the plate. Any antibody
specific for the antigen will bind to the available sites. The bound antibody
was detected by incubation with an enzyme labeled specific anti-immunoglobulin
followed by the enzyme substrate.
Indirect ELISA using W.bancrofti
mf ES antigen, incubated with filarial sera samples followed by anti-human
IgG-penicillinase conjugate and starch-iodine penicillin substrate was found to
be highly sensitive in detection of filarial infection. As little as 0.35 ng
antigen protein per well was found to be sufficient in detecting filarial
antibody compared to 0.1-1.5 µg of soluble mf antigen protein per well used
earlier (13).
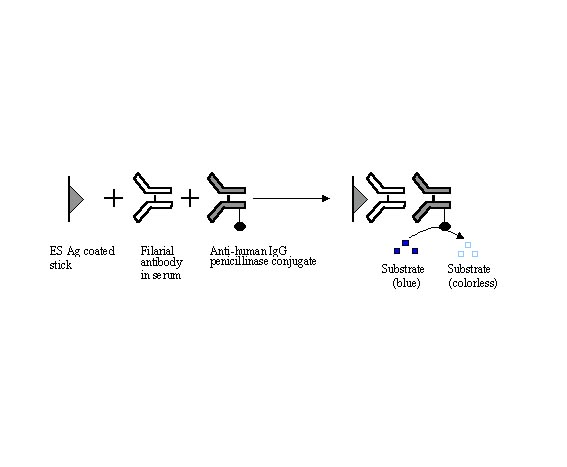
Fig. 1- Indirect ELISA
The
ELISA plates are to be imported and costly. Also the antigen bound on plate may
vary. Hence stick ELISA was developed with cellulose acetate membrane (CAM)
square fixed onto a strip of used X-ray sheet. This has become simpler and
economic. In indirect ELISA about 100 pg of Wbmf ES antigen protein was found to
be sufficient per test and thus one ml of culture fluid can be used for about 2
million tests (14). Brugia malayi
microfilarial excretory-secretory antigens have been shown to be very useful in
detection of acute, clinical and occult filarial infections by stick indirect
ELISA (42). Indirect plate peroxidase ELISA using the same antigen has also been
shown to be very useful to detect active filarial infection with high
specificity of 95% and sensitivity of 68%. (15). Indirect ELISA using CAM has
also been developed to detect tuberculous IgG antibodies against a purified
31kDa antigen (ES-31) from M. tuberculosis
H37Ra (16,17). Dot ELISA using nitrocellulose membrane has been
used for the diagnosis of visceral leishmaniasis (18) and filariasis (19, 20).
Direct ELISA
Direct ELISA was used in our laboratory for detection of antigen in
filarial immunecomplexes. Optimal dilution of circulating immunecomplexes (10
µg/ ml) was coated on to the plate. After washing, filarial serum
immunoglobulin G-penicillinase (FSIgG-penicillinase) conjugate was added and
then assayed. Analysis of immune complexes showed the presence of filarial
antigen in 30 out of 33 clinical filarial, 2 out of 15 endemic normal and none
of the non endemic normal sera (21).
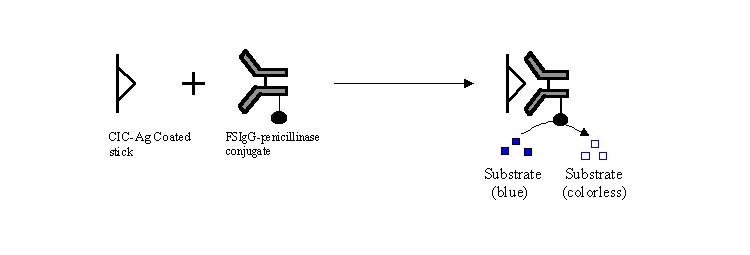
Competitive
ELISA
Competitive ELISA is useful for identification and quantitation of either
antigen or antibody. In antigen determination by this method, antigen present in
the sample competes for sites on the antibody with labeled antigen added to the
medium. The color change will be inversely proportional to the amount of antigen
in the sample. Competition principle can be exploited in number of ways.
To identify the antigen involved in filarial immunecomplexes, circulating
immunecomplex (CICs) showing filarial antigen in direct ELISA was coated on to
the plates. Then 50 µl of culture fluid containing W.
bancrofti mf ES antigen (7ng/ml) was added together with 50 µl of FSIgG
penicillinase conjugate. After incubation and washing, the enzyme
activity was assayed. In this assay system, freely added W.bancrofti
mf ES Ag competes with the immune-complexed antigen bound to the plate, for
binding sites on FSIgG conjugated with penicillinase. Presence of W.bancrofti mf ES antigen in CICs was confirmed by the persistence
of blue color (21).
Fig.
3- Competitive ELISA
Sandwich ELISA
Double antibody sandwich ELISA is useful for detection of antigen. In the
assay system the antigen to be detected is sandwiched between two similar or
different antibodies of which one is labeled with an enzyme. IgG fraction of
human filarial serum immunoglobulin (FSIgG) has been successfully used for
detection of circulating filarial antigen by sandwich ELISA (22). When analysed
using FSIgG sandwich ELISA, 27 out
of 33 microfilaraemia, 19 out of 30 clinical filarial and none of the 20
non-endemic normal sera showed the presence of filarial antigen. Detection of
filarial antigen using FSIgG in sandwich ELISA showed an apparent positive
correlation between microfilarial density and antigen titer. When Wb E34
monoclonal antibody was used along with FSIgG in double antibody sandwich ELISA
68% of microfilaraemic sera showed the presence of filarial antigen (23).
Detection of filarial antigen in urine and hydrocele fluid samples by sandwich
ELISA using FSIgG was also reported (24,25). Stick sandwich ELISA was also
developed for detection of circulating free antigen and CIC-antigen in
tuberculosis sera (26).
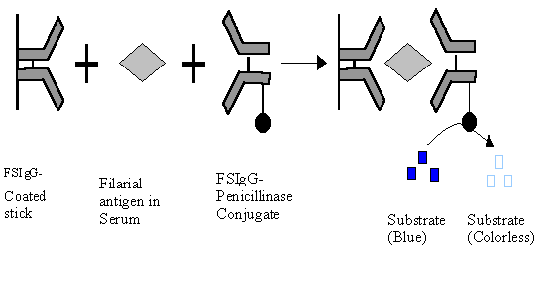
Fig. 4- Sandwich ELISA
Inhibition ELISA
Inhibition ELISA works similar to competitive ELISA but in this system
the two antigens (antigen in test sample and enzyme labeled antigen) are added
one after another. This is useful especially when test serum contains both
antigen and antibody of interest in the immune reaction. Inhibition ELISA was
also useful in determining the identity of specific antigen or antibody (27,28).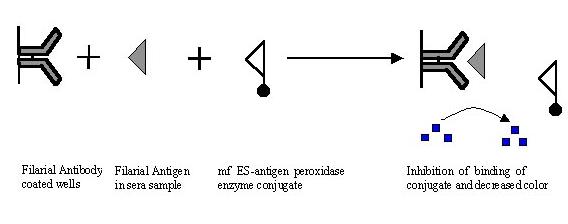
Fig-
5.
Inhibition ELISA
Inhibition
ELISA was found to be better than sandwich ELISA in detection of active filarial
infection (27). FSIgG was coated to the plate followed by addition of test serum
containing antigen. After washing Wb mf ES antigen conjugated with penicillinase
was added. Positive reaction for ES antigen was indicated by the persistence of
the blue color. By inhibition ELISA using Wb mf ES Ag-penicillinase conjugate,
filarial antigen was detected in about 90% of microfilaraemic sera and 60% of
clinical filarial sera. Incorporation of avidin-biotin system in inhibition
ELISA increased the sensitivity of the assay system by 67% decrease in the false
negative results (29). Stick inhibition ELISA using B.
malayi mf ES antigen- penicillinase and FSIgG was found to be useful to
detect filarial antigen in acute, chronic and occult filarial cases where mf
cannot be detected in peripheral circulation. While the filarial antigen
positivity ranged between 43-67% in lymphadenopathy cases, as many as 70% of
epididymoorchitis cases, 61-62% of TPE & tenosynovitis cases and 80% of
retroperitoneal lymphadenitis cases showed antigenemia (30). Filarial sera after
acid-heat treatment for dissociation of immunecomplexes have been analysed in
inhibition ELISA to detect immunecomplexed filarial antigen (31).
Anti C3 ELISA:
Anti C3
ELISA is useful to detect disease specific circulating immunecomplexes or
specific antigen present in circulating immune-complexes. Test sera are
incubated in microtitre plate wells prior sensitized with rabbit/goat anti human
C3 antibody. The filarial antigen in bound immunecomplexes is
detected by further incubating the wells with enzyme labeled specific antibody.
In our laboratory we have coated anti C3
antibody (0.1 µg/100 µl/well), blocked with 3% BSA and further incubated with
optimally diluted (1:150) test sera. FSIgG penicillinase conjugate was used as a
probe to detect the filarial specific antigen in bound immunecomplexes. Sera
from clinical filarial patients (10 years or more duration) were negative for
free circulating antigen but showed high level of immunecomplexed antigen (32)
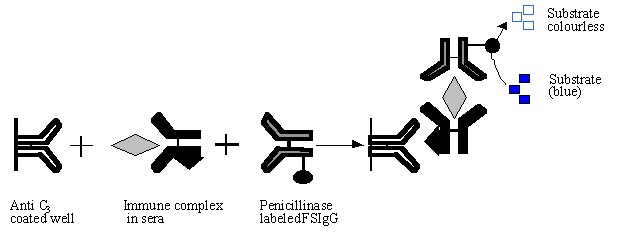
Fig 6. Anti C3 ELISA
Immunodiagnosis
of filarial infection
Immunodiagnosis of
filarial infection
still presents
a major
challenge to
the immunoparasitologist because
of the complexities of
the disease
and the
high degree
of cross-reactivity between filaria and other nematode species. The
sequential changes in the humoral immune response against filarial antigens
during the course of Brugia malayi infection in Mastomys
natalensis have been studied using
penicillinase ELISA which was useful in understanding immune recognition and
in assessing the status of host immunity to invading parasite(33).Stick ELISA
using penicillinase system was used for antigen, antibody and immunecomplex
detection in sera of patients who were in different stages of bancroftian
filarial infection and found to be useful in understanding disease status and
its relevance to immunopathogenesis. The diagnostic assay system based on
PEN-ELISA using Wb mf ES antigen for the detection of filarial antigen and
antibody was found to be one of the three promising assay systems for diagnosis
of filariasis in the multi-centre trial held world wide by WHO-TDR programme
(W.F. Piessens, personal communication). Blood collected on filter paper by
finger prick can also be used in ELISA making it easily adaptable for field
studies (34). Field evaluation of PEN-ELISA using Wb mf ES Ag for the detection
of filarial antibody in filter paper blood samples showed the sensitivity and
specificity of about 80-90 % in diagnosis of filarial infection (35). It was
observed in our laboratory that 0.5 femtogram of antigen isolated from filarial
patients urine is sufficient per test for detection of antibody (36). Twenty
four hours urine from a carrier can give sufficient antigen for 600 billion
tests. Thus it has been proved in our hands and in this laboratory, that the
more you sharpen penicillinase ELISA with specific reagent the more efficient it
works. B. malayi mf
ES antigens have been shown to be very useful in detection of acute,
clinical and occult filarial infections. Filarial IgG antibodies against Bm
mf ES antigen were detected in 54-72% of acute lymphadenopathy cases, 67-84%
of chronic filarial cases and 44-66% of occult filarial cases that included
cases of tropical pulmonary eosinophilia (TPE),
mono or poly arthritis etc.(30). Recently, B.malayi microfilarial ES antigen and Filarial serum
Immunoglobulins have been used to pick up 2/7 and 5/7 of hematuria cases with
filarial etiology respectively (37). While a number of immunoassays such as the
ICT kit and Og4C3 ELISA have been used, developed based on filarial antigen
detection using monoclonal antibodies (38,39), these assays are capable of
detecting microfilaraemic cases and failed to detect acute and early filarial
cases. A study evaluating Seva-Fila
Chek and the ICT kit showed a much greater sensitivity of the former in
detecting clinical and occult cases (40).
Immunomonitoring of filarial infection
Although mass or targeted administration of diethylcarbamazine (DEC) has
been the regular procedure of filariasis control programmes, the logistics of
such programme are greatly complicated by the haphazard result in total
clearance of microfilariae. A long term study on the effect of chemotherapy in
microfilaraemic patients and immunomonitoring
of filarial patients in an endemic area will be useful in developing an
immunological parameter in place of parasite detection, determining appropriate
duration of treatment and to judge the effectiveness of chemotherapy and relapse
of infection or reinfection in control programme in an endemic area. A group of
27 W. bancrofti microfilaraemic cases were treated with DEC and
followed up for 10 years (41). Treatment
with DEC was for 14 days (day 1, 1 mg Kg-1 body weight; day 2, 2 mg Kg-1 body weight and from day 3 onwards 6 mg Kg-1
body weight) followed by one dose (6 mg Kg-1
body weight) each year. Out of the 27 carriers followed, 13 cases never showed
reappearance of mf while 14 cases showed reappearance of mf in the peripheral
blood during the study period. Immunomonitoring showed decrease in antigen and
antibody levels during therapy followed by increased titres with recurrence or
re-infection in few cases in an endemic area suggesting use of antigen/antibody
assay as a marker for infection. None of the cases under DEC treatment developed
any clinical symptoms during the study.
Further a total of 38 filarial cases with acute, chronic and occult
clinical symptoms were given a long term treatment with DEC (6 mg Kg-1 body weight per day for 21 days in a month repeated for 3 to
12 months) and were monitored by immunological tests for antigen and antibody
(42). A significant number of filarial patients showed a reversal or reduction
in the clinical symptoms. The immunomonitoring thus helped in deciding the
appropriate period of treatment in the absence of microfilaraemia in these
clinical conditions. Immunomonitoring followed by opDEC (optimal DEC) therapy
was also found to be very effective for clinical relief and cure (43) in such
cases. In another report from this laboratory (44) number of filaria associated
clinical manifestations have been shown in children in an endemic area. These
cases with manifestations like lymphadenopathy, asthmatic bronchitis, pulmonary
eosinophilia, monoarthritis, recurrent URI, pneumonia, pain in abdomen etc. were
effectively managed by immunomonitoring and optimal DEC (op DEC) therapy.
The
PEN-ELISA thus has been explored in a variety of ways to detect, identify and
quantitate antibodies, free and immunecomplexed antigen of interest in
diagnosis, protection, immunomonitoring and immunosurveillance of filariasis.
Immunodiagnosis of
tuberculosis
Tuberculosis continues to be a major public health problem in India and
other developing countries. Operational difficulties in control programmes, and
coexistence with HIV infection have contributed to the increased burden of the
disease. Early diagnosis of tuberculosis is an essential requirement for
initiating prompt treatment and containment of the disease. Presently available
methods like radiological and bacteriological examination are insensitive and
even intradermal tuberculin test cannot differentiate active and past infection.
In our laboratory Mycobacterium
tuberculosis H37Ra excretory-secretory (M.tb
ES) antigen was isolated from logarithmic growth phase of the bacilli in
synthetic Sauton medium and explored for detection of tuberculous antibodies by
Penicillinase ELISA (45). This assay was also shown to be useful for detection
of pulmonary tuberculosis and extrapulmonary tuberculosis in children (46). A
comparative analysis of M.tb ES
antigen and other tubercular antigens Viz., phosphate buffer saline soluble
(PBS-S) antigen, SDS soluble antigen (SDS-S), and PPD showed M.tb
ES antigen to be superior to other antigens in diagnosis of tuberculosis (47).
Two purified antigens from M.tb ES
antigen i.e. ES-31 (a 31 kDa protein obtained by ammonium sulphate, SDS-PAGE and
FPLC fractionation using Resource ‘S’ column) and ES-41 (a 41 kDa protein
isolated by trichloroacetate solubilization followed by FPLC fractionation using
Superdex HR 10/30 gel filtration and Resource ‘S’ columns) were shown to be
of immunodiagnostic importance in tuberculosis (16,17,48,49). ES-31 antigen was
useful to detect tuberculous IgG antibodies in 92% of pulmonary and 88%
tuberculous lymphadenopathy and 90% of tuberculosis meningitis cases, while the
ES-41 antigen detected antibodies in 82% of abdominal tuberculosis and 85%
osteoarticular tuberculosis cases. A
process of isolation and purification of M.tb
ES-31 protein and monitoring of M.
tuberculosis infection has been patented. A sandwich ELISA using affinity purified goat anti ES-31
antibodies was found to be useful to detect tubercular free antigen and
immunecomplexed antigen in 80% and 72% of pulmonary tuberculosis cases respectively
(26).
Immunodiagnosis of Leprosy
Leprosy is another widely distributed Tropical disease. Studies have been
initiated in our laboratory to develop suitable immunoassays for the early
diagnosis of leprosy and for early detection of nerve damage in affected cases.
Anti ceramide antibodies and S-100 protein levels were estimated in leprosy
patients (50, 51). S-100 antigen protein was reported as more sensitive and
reliable marker than anti ceramide antibodies for nerve damage (50). The
increase in levels of IgM anti ceramide antibody in the tuberculoid group of
patients with reaction, when compared to those without reaction, is significant
(P<0.05). Similarly, significant increase (P<0.01) was observed in the
borderline group with reaction (51).
References:
1. Engvall &
Perlmann P. (1971). Enzyme Linked immunosorbant assay (ELISA): Quantitative
assay for immunogobulin. Immunochemistry. 8. 871.
2. Voller A, Bidwell DE
and Bartlett A (1979) The enzyme linked immunosorbent assay (ELISA). A guide
with abstracts of microplate applications. Dynatech Europe publication.
3. Talwar G.P (1983)
Non-isotopic immunoassays and their applications. Vikas Publishing House Pvt.
Ltd., New Delhi.
4. Desai N., KhatKhatay
I, Sankolli G, Neherji P and Joshi U (1989) Enzyme labeled
immunoassay for urinary gonadotropins using penicllinase, Clinica Chemica
Acta. 184,315.
5. Shrivastav TG, Kumari
GL and Rao PN (1988) Enzyme immunoassay of cortisol in human plasma using
penicillinase label. Clinica Chemica Acta, 174,83.
6. Sauar NJ, Foulkes JA
and O’Neill PM (1989) A comparison of alkaline phosphatase,
ß-galactosidase, penicillinase and peroxidase used as labels for
progesterone determination in milk by heterologous microtiter plate enzyme
immunoassays. J. Steroid Biochem. 33,423.
7. Novick RP (1962)
Micro-iodometric assay for penicillinase, Biochem. J., 83, 236.
8. Yolken RH. Wee SB,
Regenmortel MV (1984) The use of B-lactamase in enzyme
immunoassays for detection of microbial antigens J. Immunol. Methods, 73,
109.
9. Harinath BC (1984)
Immunodiagnosis of bancroftian filariasis-problems and progress. J.Biosci., 6.
691.
10. Harinath BC (1986) Detection and
diagnostic utility of in vitro and in vivo released antigens in bancroftian filariasis. J. Comm. Dis.,
18, 261.
11. Ramaprasad P, Kharat I and
Harinath BC (1984) Comparative efficiency of penicillinase ELISA and RIPEGA
using 14C -labeled Wuchereria bancrofti
excretory-secretory antigen for the detection of filarial antibody. IRCS
Med. Sci, 12, 738.
12. Ramaprasad P, Reddy MVR, Kharat I
and Harinath BC (1985). Comparison of radio
immunoassay and inhibition enzyme linked immunosorbent assay (ELISA) using 14C-labeled
Wuchereria bancrofti microfilarial
excretory-secretory antigen for the detection of filarial antigen. IRCS Med. Sci,
13, 1110.
13. Kharat I, Ghirnikar SN and
Harinath BC (1982) Antibody analysis in human filarial sera by ELISA using Wuchereria bancrofti microfilariae culture antigen. Indian
J.Exp.Biol, 20, 378.
14. Parkhe KA, Prasad GBKS, Das A,
Harinath BC, Roebber N and Hamilton RG (1986) Dics/stick ELISA for diagnosis of
bancroftian filariasis, Indian J. Exp. Biol, 24, 437.
15. MVR Reddy, R Alli, KK Devi, R
Narayan, R Harikrishnan, K Cheirmaraj and BC Harinath. Comparative evaluation of
microtitre plate peroxidase and
Stick Penicillinase enzyme immunoassays for detection of filarial antibodies
using Brugia malayi microfilarial excretory- secretory antigen. J. Parasitic.
Dis. 1996, 20, 173-176.
16. Nair ER, Kumar S, Reddy MVR and
Harinath BC (1998) Mycobacterium
tuberculosis H37 Ra ESAS-7 an excretory - secretory antigen fraction of
immunodiagnostic potential in pulmonary tuberculosis; Indian J Clin. Biochem 13
(2), 98-105.
17. Nair ER, Banerjee S, Kumar S, and
Harinath BC, 2000. Isolation and characterization of a 31 kDa mycobacterial
antigen from tuberculosis sera and its identification with in
vitro released culture filtrate
antigen of M.tb H37
Ra bacilli; Scand J Infect Dis. 32: 551-556.
18. Pappas MG, Hajkowshi R and
Hockmeyer WT (1983) Dot enzyme linked immunosorbent assay (Dot-ELISA): a micro
technique for the rapid diagnosis of visceral leishmaniasis. J. Immunol.
Methods, 64, 205.
19. Tandon A, Murthy PK, Saxena RP,
Sen AB and Saxena KC (1988) Dot-ELISA for
diagnosis of lymphatic filariasis. Indian J. Med. Res. 87, 429.
20. Balaji Ganesh B, Kader AM,
Agarwal GS, Reddy MVR and Harinath BC (2001) A simple & inexpensive dot blot
assay using a 66 kDa B. malayi
microfilarial antigen protein for diagnosis of filarial infection in an endemic
area. Trans. Roy. Soc. Trop. Med. Hyg. 2001; 95:
1-2.
21. Prasad GBKS, Reddy MVR and
Harinath BC (1983) Detection of filarial antigen in immunecomplexes in
bancroftian filariasis by ELISA. Indian J. Med. Res, 78, 780.
22. Reddy MVR, Malhotra A and
Harinath BC (1984) Detection of circulating antigen in bancroftian filariasis by
sandwich ELISA using filarial serum IgG. J.
Helminthol, 58, 259.
23. Reddy MVR, Ramaprasad P, Piessens
WF and Harinath BC (1986) Diagnostic utility of monoclonal antibodies raised
against microfilarial excretory-secretory antigens in bancroftian filariasis. J.
Biosci, 10, 461.
24. Malhotra A, Reddy MVR and
Harinath BC (1985) Detection of filarial
antigen in urine by sandwich ELISA and its use in diagnosis. Indian J.
Med. Res, 81, 123.
25. Malhotra A, Prasad GBKS and
Harinath BC (1985) Detection and isolation of filarial antigen from hydrocele
fluid and its use in diagnosis. Indian
J. Exp. Biol, 23, 180.
26. S Banerjee, E Nair, S Kumar, MVR
Reddy & BC Harinath. Assay of tubercular antibody, circulating free and
immune complexed antigen in the diagnosis of pulmonary
tuberculosis. Ind. J. Clin. Biochem, 2001, 16(2), 203-206.
27. Malhotra A and Harinath BC (1984)
Detection and monitoring of Microfilarial
ES antigen level by inhibition ELISA during DEC therapy. Indian J. Med. Res, 79,
194.
28. Reddy MVR, Parkhe KA, Piessens WF
and Harinath BC (1989) Wb E34 monoclonal
antibody: Further characterization and diagnostic use in bancroftian filariasis.
J.Clin. Lab. Analysis, 3, 277.
29. Parkhe KA, Ramaprasad P and
Harinath BC (1988) Stick enzyme linked immunosorbent assay using the avidin-biotin
system for detection of circulating antigen in bancroftian filariasis. J. Biosci,
13, 229.
30. Harinath BC & Reddy MVR.
(1997) Diagnosis & Immunomonitoring in successful management
of bancroftian filariasis. J. of Parasitic Diseases, 21, 41-51.
32. Alilkhan A, Parkhe KA, Reddy MVR
and Harinath BC (1990) Filarial antigen, antibody and circulating
immunecomplexed antigen level in bancroftian filariasis by Stick ELISA. Natl.
Med. J. India, 3, 265.
33. Cheirmaraj K and Harinath BC
(1991) Humoral immune response to infective larval antigen in Brugia
malayi infected Mastomys natalensis.
Acta Tropica, 48, 305.
34. Malhotra A, Reddy MVR, Naidu JN,
Ghirnikar SN and Harinath BC (1982) Detection of filarial infection using Wuchereria bancrofti
microfilariae culture antigen and filter paper blood samples in enzyme linked
immunosorbent assay. J. Blosci., 4. 507.
35. Harinath BC, Malhotra A,
Ghirnikar SN, Annadate SD, Issacs VP and Bharati KS (1984) Field evaluation of
ELISA using Wuchereria bancrofti Mf ES antigen for bancroftian filariasis.
Bull. WHO, 62, 941.
36. Ramaprasad P, Kharat I and
Harinath BC (1987) Fractionation and characterization of
urinary filarial antigen. Asian Pacific J. Aller Immunol, 5, 173.
37. R.Alli, B. Bhunia, M.V.R.Reddy
& B.C. Harinath. Microscopic haematuria as an occult filarial infection in
an endemic area for bancroftian filariasis. Ind. J. Clin. Biochem. (2002). (In
Communication).
38. G J Weil, D C Santhanam, A
Malhotra , H Kumar, K V P Sethumadhavan, F Liftis and TK Ghosh. A monoclonal
antibody based enzyme immunoassay for detecting parasite antigenemia in
bancroftian filariasis. J. Inf. Dis., 1987, 156: 35- 355.
39. SJ Moore and DB Copeman. A highly
specific and sensitive monoclonal antibody based ELISA for the detection of
circulating antigen in bancroftian filariasis. Trop. Med. Parasitol., 1990, 41,
403-406 .
40. R Alli, S Kulkarni, MVR Reddy and
BC Harinath. Evaluation of Seva Fila Chek immunoassays
and rapid ICT- filariasis test for detection of bancroftian filariasis Ind. J.
Clin. Biochem, 2001, 16(2), 207-210.
41. Padigel U M, Reddy M V R, Ali
Khan A, and Harinath B C (1995). Immunomonitoring of filarial patients during
DEC therapy in an endemic area: a seven year follow up. J. Trop Med Hyg. 98.52.
42. Harinath BC, Padigel UM, Reddy
MVR & Devi KK (1996). Diagnosis and immunomonitoring in management of
lymphatic filariasis in an endemic area. J. Pars. Dis. 20, 35-40.
43. Harinath BC, Reddy MVR, Alli R,
Mehta VK, Chaturvedi P, Patond KR, Kalantri SP & Gupta RKC (1999)
Immunomonitoring followed by optimal DEC therapy for successful management of
clinical filariasis in an endemic area. Ind. J. Clin. Bio. Chem. 14 (2),
100-108.
44. Harinath BC, Reddy MVR, Bhunia B,
Bhandari YP, Mehta VK, Chaturvedi P, Prajapati N C & Gupta RKC (2000).
Filaria associated clinical manifestations in children in an endemic area &
morbidity control by immunomonitoring & optimal DEC therapy: Sevagram
experience. Ind. J. of clin. Bio.
Chem. 15 (Suppl): 118-126.
45. Basak A, Sinha Choudhary S, Lodam
A.N, Gupta OP, Narang P, and Harinath B C. (1994). Detection of IgG using Mycobacterium tuberculosis H37
Ra ES antigen in tuberculosis by Penicillinase stick ELISA. Proceedings of CSIR
Golden Jubilee symposium on tropical diseases “Molecular Biology and control
strategies”. 558.
46. Bhaskar A, Pradan P, Chaturvedi
P, Basak A, Lodam A N, Narang P, and Harinath BC, (1994). Immunodiagnosis of
childhood pulmonary and extrapulmonary tuberculosis using mycobacterium ES
antigen by Penicillinase ELISA. Annals of Trop Pead. 14, 25.
47. Satish Kumar, Chenthamarakshan V,
Reddy M V R, Narang P, Gupta OP and Harinath B C., (1994) Detection of
tuberculous IgG antibodies using Mycobacterium
tuberculosis H37 Ra Excretory Secretory antigen and tuberculous purified
protein derivative. Ind. J. Exptl Biol
32. 163.
48. S Banerjee, S Gupta, S Kumar, A J
Shrikhande, M V R Reddy & BC Harinath. Seroreactivity of 31 kDa and 41 kDa
mycobacterial secretory proteins isolated from culture filtrate in
extrapulmonary tuberculosis. Ind. J. Pathol Microbiol. ( In press)
49. S Banerjee, E Nair, S Kumar &
BC Harinath. Isolation and characterization of in
vivo
released 41kDa mycobacterial
antigen in pulmonary and bone and joint tuberculosis and its identification with
H37Ra in vitro released antigen. Int. J. Tuberculosis and lung disease
(In Press).
50. Narayan R, Maheshwari PK, Desikan
KV and Harinath BC (1997) Detection of S-100
protein and anticeramides antibodies in leprosy patients by ELISA. Lepra
Rev 68.
51. Narayan R, Maheshwari PK, Desikan
KV and Harinath BC. Detection of S-100 antigen and anticeramides antibody in
sera of leprosy patients with and without reaction. Ind. J. of Lepr. 69 (4),
347-352.
This
review is broadly based on the work done at
Department
of Biochemistry
Jamnalal
Bajaj Tropical Disease Research Centre,
Mahatma Gandhi Institute of Medical Sciences, Sevagram, India 442 102.