13. Western
Blot Analysis
A. Principle :
The
Western blot analysis, first described by Towbin et al. (1979) is an
extremely sensitive method for visualizing specific proteins in complex antigenic
mixtures. In this technique, the separated protein components in SDS-PAGE are
transferred onto the surface of nitrocellulose
sheet for immobilization and then identified based on their
immunological reaction with antibody and enzyme labelled or
radio-iodinated second antibody probe. This technique is useful for comparing
reactivity of several sera with a single antigenic preparation or of a single
antibody preparation with several antigen sources.
B. Materials :
a.
Transblotting apparatus
with power supply and gel cassettes.
b.
Nitrocellulose membrane (Schleicher
and Schuell, BA 85, 0.45 m pore size)
c.
Whatman filter paper No.3
d. Culture tubes with screw
caps.
e.
Water bath with shaker.
f. ransfer buffer (Tris
glycine buffer, pH 8.3)
(Appendix-1)
g. 0.25 M SPB, pH 7.2
(Appendix-1)
h. Quench solution (5% skimmed milk powder in PBS)
Dissolve 10g skimmed milk powder (SMP) in 200 ml
P
i. Washing buffer
(PBS/T) (Appendix-I)
j. Citrate-Phosphate
buffer (pH 5.0) (Appendix-I)
k. Diaminobenzidine substrate
(Appendix-I)
l. Anti-human IgG
peroxidase conjugate.
m. Transbloting buffer
(Appendix-I)
C. Method :
a)
Pre-cooling of transfer buffer and
preparation of gel and nitrocellulose paper (NCP)
1.
Pour 5
litres of
transfer buffer
into the transblotting apparatus tank and keep in cold
atleast 4 hrs before transblotting for precooling.
2.
After SDS-PAGE run is over, incubate the gel in transfer buffer for 30
minutes and then change the gel
into fresh transfer buffer and incubate for another 30 minutes. This will
eliminate the possible swelling of the gel during transblotting.
3.
During the second 30 minutes incubation of gel, cut a piece of NCP
slightly larger than the gel and wet it by rolling onto distilled water. When
completely moist, submerge for 5-10
minutes and then place it in transfer buffer to equilibrate for 15 minutes.
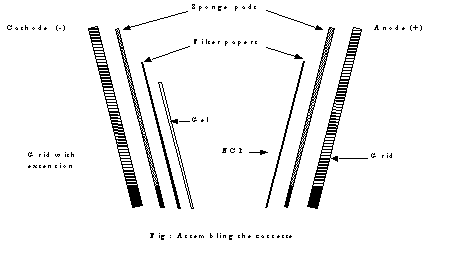
b)
Assembling the cassette
1.
Pour one litre of transfer buffer into a tray large enough to hold the
gel and cassette to a depth of about one inch.
2.
Place the grid with molded extension
in tray so that pins face upwards.
3.
Place the
dacron sponge
on it
using the
pins as
a guide
and press carefully on the
sponge to avoid any trapped air bubbles.
4.
Cut off molecular weight standards lane and reference lanes, if
any, from
the gel for protein
staining. Notch the gel for orientation.
5.
Cut 4 Whatman 3 filter papers slightly larger than the gel and wet by
placing them in Transfer buffer.
6.
Pick up the gel with two wet filter papers and place on the sponge with
the filter paper next to the sponge.
7.
Cover with
NCP, dull
side in contact with gel and
mark NCP overhang with pencil to mark the orientation.
8.
Place a second piece of Whatman 3 filter paper, sponge pad and grid.
Recheck the order of layers which should be grid with extension-sponge
pad-filter paper-gel - NCP - filter paper-sponge pad-grid without extension.
9.
Close the cassette by matching the clips in one half with the holes in
the other. Slide the top grid
down slightly
into the
grooves on
the clips until it holds firmly in place, if necessary
rubber bands
can be
used to
apply additional
pressure to
the ends of the cassette.
c)
Transfer
1.
Place the assembled cassette in the buffer tank with the gel side (i.e.
the side with grid having extension) facing the cathode and the NCP side (i.e.
the side the grid having no extension) facing the anode.
2.
Top up
with more
transfer buffer
if necessary and place the
power supply lid on the chamber so that the anode female connector (+) on the
lid contacts the electrode which is to function as the anode.
3.
Turn the Voltage, adjust knob fully
clockwise. The current will start at 0.6 to 0.8 amps and
run for one and half hours. The current will increase to
about 1.2 amps during the run ( if
the transfer
buffer gets
hot during the run, decrease the voltage and increase the time).

4.
After the electrophoretic transfer is completed, turn the voltage, adjust
knob fully counterclockwise. Turn
off the power and unplug the lid from the main. Carefully lift the power supply
lid of the buffer chamber. Place the cassette in an empty tray with gel side
down.
Disengage the two halves and remove the sponge and filter
paper. Very carefully
remove the NCP from the gel by starting at one end and
"Peeling" it away.
5.
Place the NCP (Dull-side up) in
Quench for 2 hr at 370C or overnight
at 40C.
6.
Stain the gel with coomassie blue to
assess the transfer.
7.
After quench is complete, wash NCP four times, each time for 15 minutes
with PBS/T with 2% SMP.
8.
Cut NCP into strips for immunoblotting and identify the strips with
pencil.
d) Immunoblotting
1.
Incubate NCP strips in optimally diluted sera (1:50) or antibody solution
and controls in PBS/T with 0.5% BSA at 370C for 2 hrs (in screw cap culture tubes).
2.
Wash NCP strips in PBS/T four times, each time for 20 minutes.
3.
Incubate the strips with optimally diluted peroxidase labelled second
antibody at 370
C for 2 hrs.
4.
Wash the NCP
strips in PBS/T as described above.
5.
Place the NCP strips in freshly
prepared DAB-substrate
solution in culture tubes or petridish for about 30 minutes in reduced light.
6.
Stop the reaction by transferring
the strips to water, air dry and preserve.
Note :
1.
The dull side of NCP should be up in
all wash steps to protect from abrasion, since it will
receive the blotted antigens.
2.
Avoid air bubbles in all stages of assembling the cassette.
3.
Transblotting should preferably be done at 40C.
It can also be done at low voltage and current
for long hrs (30 V and 0.1 Amp for 24 hr).
4.
During transblotting, buffer circulation is generally not necessary.
However if desired, place a magnetic stir-bar in the bottom of tank and
carefully place the unit on magnetic stirrer but the turbulance should be
maintained at minimum.
5.
If necessary, all or part of NCP after transblotting can be stored for
future use (at least for 5 days ). The NCP
should be
incubated in
PBS with 0.01% sodium azide (after Quenching )
for 30
minutes, dried,
sealed in
a plastic
bag and then
stored at 40C.
Remember to wash thoroughly before using to remove azide if
peroxidase is used in the immunoblotting.
6.
Diaminobenzidine is
carcinogenic and
so use gloves while handling
and avoid breathing.
It is also light sensitive and should be handled in subdued light.
7.
If necessary,
stain the
NCP after transfer with 'ponceau' stain
( 2.0% ponceau S in 3%
trichloroacetic acid
in distilled
water ) for
5 minutes and destain in distilled water to assess the transfer of
proteins. After ponceau staining the NCP can be further used for immunoblotting
after quenching.
8.
If desired, after transblotting a part of the NCP can also be stained
directly using 0.2% coomassie blue R - 250,
50 % methanol,
10% acetic
acid for 5 minutes. It should be destained quickly in 90% methanol and 2%
acetic acid to avoid disintegration
of NCP.
9.
To keep the transblotting unit in good operating order, rinse the chamber
and cassettes with distilled
water and
dry immediately after the transblotting. Also clean the power supply lid
with a clean cloth and keep dry. Do not use strong detergent for cleaning.
1.
Towbin , H .
Slahelin , T .
and Bordon,
J. Electrophoretic transfer
of proteins from polyacrylamide gels
to nitro-cellulose
sheets procedure and some
applications. Proc.Nat. Acad. SC., USA, 76 (1979), 4350.
2. Genes and Antigens of parasites - A lab. Manual edited by C. LM. Moral, publishing by UNDP / Word Bank / WHO special programme for Research and Training in Tropical Diseases.